Uterine Microbiota Testing
Uterine microbiota testing
Emerging research suggests that the uterus harbors its own microbial community, similar to other body sites like the gut, skin, or oral cavity. Examination of the microbial community present in the uterus can help identify imbalances or dysbiosis in the uterine microbiome, aiding in the diagnosis and management of conditions such as endometritis, infertility, preterm birth and certain gynecological disorders.
Uterine microbiota testing, also known as uterine microbiome analysis, involves the examination of the microbial community present in the uterus. The uterine microbiota refers to the diverse collection of microorganisms, including bacteria, viruses, fungi, and other microbes, that inhabit the uterine cavity.
Traditionally, it was believed that the uterus was a sterile environment. However, recent research has revealed the presence of a low-abundance, diverse microbial community in the uterus, similar to other parts of the reproductive tract. The study of the uterine microbiota is still a relatively new field, and scientists are exploring its role in various reproductive health conditions and pregnancy outcomes.
Uterine microbiota testing typically involves collecting samples from the uterine cavity using minimally invasive techniques such as transcervical swabs or aspirates. These samples are then analyzed using advanced molecular techniques, such as high-throughput DNA sequencing, to identify and characterize the microorganisms present in the sample.
The information obtained from uterine microbiota testing can provide insights into the composition, diversity, and relative abundance of different microbial species within the uterus. Researchers are particularly interested in understanding how alterations in the uterine microbiota may be associated with conditions such as endometritis (inflammation of the uterine lining), infertility, pregnancy complications, and certain gynecological disorders.
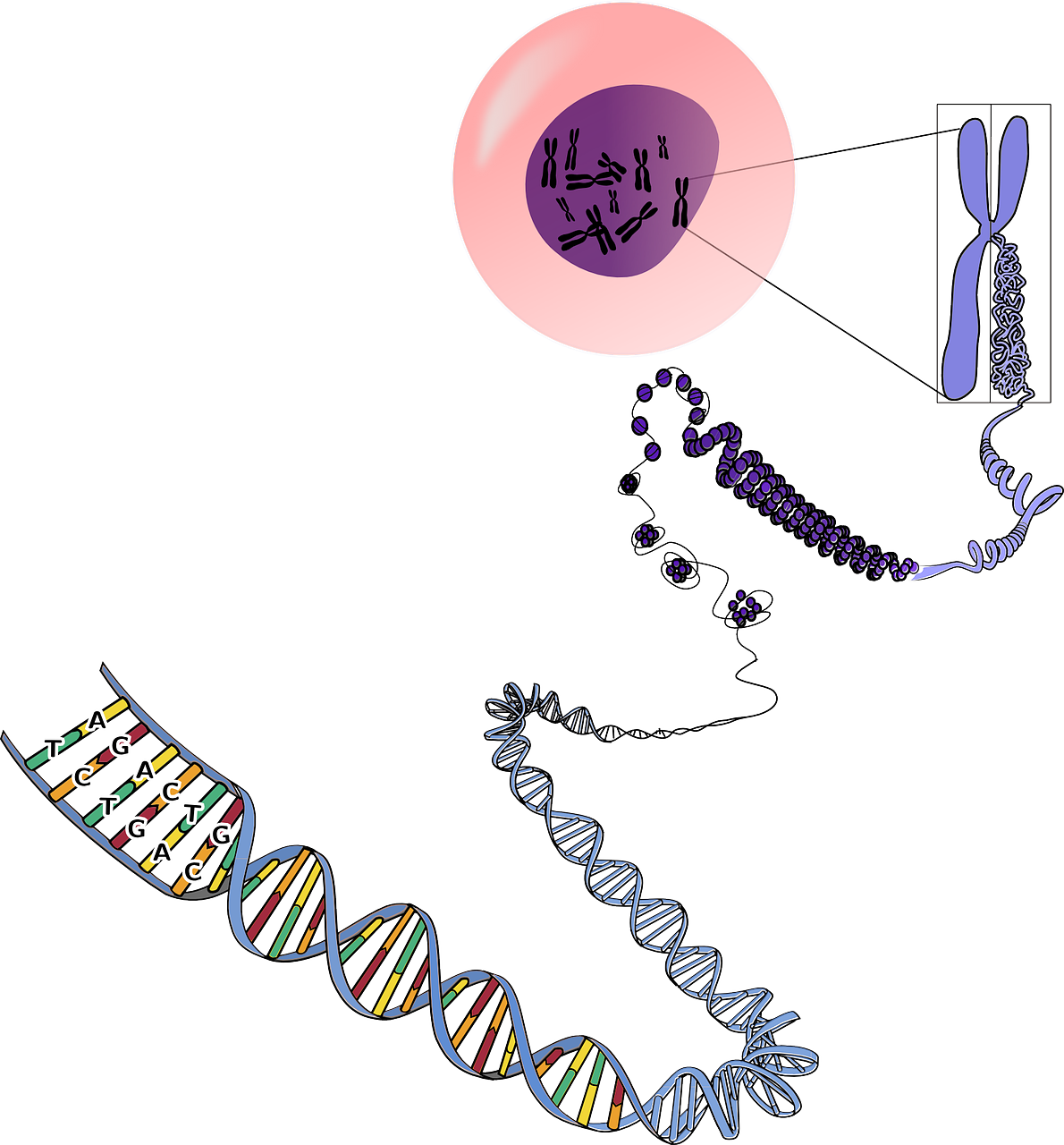
What is DNA Sequencing?
DNA sequencing is the process of determining the precise order or sequence of nucleotides (adenine, thymine, cytosine, and guanine) in a DNA molecule. It provides a detailed blueprint of an organism’s genetic information encoded within its DNA.
There are different methods and technologies for DNA sequencing, but the basic principle involves breaking the DNA into smaller fragments, determining the sequence of each fragment, and then piecing the fragments together to reconstruct the entire DNA sequence.
The most widely used method for DNA sequencing until relatively recently was Sanger sequencing, developed by Frederick Sanger in the 1970s. In recent years this method has been almost entirely superseded by Next-Generation Sequencing (NGS) technologies that have revolutionized DNA sequencing.
DNA sequencing plays a fundamental role in advancing our understanding of genetics and genomics and has significant implications for various aspects of biology and medicine.
What is Next-Generation Sequencing?
Next-Generation Sequencing (NGS) is a revolutionary technology which enables determination of the precise sequence of DNA for thousands to millions of DNA molecules simultaneously. It has transformed the field of genomics by enabling high-throughput, cost-effective, and scalable sequencing capabilities, enabling a wide range of applications in clinical diagnostics, genetic diseases and personalised medicine as well as driving significant advancements in various fields of biology and medicine. Using NGS, an entire human genome can be sequenced within a single day, compared to over a decade required by the previous sequencing technology (Sanger sequencing).
There are a number of different NGS platforms using different sequencing technologies to perform sequencing of millions of small fragments of DNA in parallel. The common principle involves breaking the genomic material into small fragments, attaching specific adapters to each fragment, and amplifying the fragments to create a cluster of identical fragments. These clusters are then sequenced simultaneously using methods such as sequencing by synthesis, sequencing by ligation, or nanopore sequencing.
The sequencing data generated by NGS platforms consists of short DNA or RNA reads, typically ranging from tens to hundreds of base pairs in length. Bioinformatics tools are used to align and assemble these reads into longer contiguous sequences called contigs, which can then be further analyzed to decipher the genetic information contained within the sample.
NGS has numerous applications in genomics research and clinical diagnostics. It has enabled the sequencing of complete genomes, identification of genetic variants associated with diseases, transcriptome analysis to study gene expression, metagenomics to explore microbial communities, epigenomics to investigate DNA modifications, and much more. The technology continues to evolve rapidly, driving advancements in various fields of biological research and personalized medicine.
5 advantages of NGS testing
NGS-based uterine microbiome testing offers several advantages over traditional culture-based methods.
1. Comphrensive assessment
Culture-based methods rely on growing microorganisms under lab conditions, which can be challenging for certain bacteria that are difficult to culture or require specific growth conditions. NGS, however, provides a culture-independent approach, allowing for a more comprehensive assessment of the uterine microbiome. It can detect a wider range of microorganisms including unculturable or low-abundance bacteria and therefore provides a more comprehensive picture of the microbial community.
![]()
2. High-resolution profiling
NGS enables high-resolution profiling of the uterine microbiome. It can identify and quantify the relative abundance of different bacterial species present, providing a detailed understanding of the microbial composition. In contrast, culture-based methods often rely on isolating and identifying dominant or easily culturable microorganisms, which may not represent the entire microbial community.
![]()
3. Unbiased analysis
Culture-based methods can be biased towards the detection and growth of certain microorganisms, potentially leading to an incomplete or skewed representation of the uterine microbiome. NGS, being a culture-independent method, does not have these biases and allows for an unbiased analysis of the microbial composition.
![]()
4. Functional analysis
NGS-based uterine microbiome testing not only provides information about the microbial composition but also offers opportunities for functional analysis. Metagenomic sequencing can provide insights into the functional capabilities and potential interactions of the uterine microbiome. This can help in understanding the role of specific microorganisms or pathways in reproductive health or disease.
![]()
5. Longitudinal monitoring
NGS enables monitoring of the uterine microbiome over time. By analyzing multiple samples from the same individual, dynamic changes can be seen in the microbial composition which can help identify specific conditions or evaluate the effectiveness of medical interventions.
![]()
These advantages contribute to a deeper understanding of the uterine microbiome and its role in reproductive health and disease.
The process of uterine microbiome testing using Next-Generation Sequencing (NGS)
Uterine microbiome testing using Next-Generation Sequencing (NGS) is a powerful technique for studying the microbial composition of the uterus.
Uterine microbiome testing using Next-Generation Sequencing (NGS) is a powerful technique for studying the microbial composition of the uterus. NGS allows for high-throughput sequencing of DNA samples, providing detailed information about the types and relative abundances of microorganisms present in the uterine microbiota.
The process of uterine microbiome testing using NGS typically involves the following steps:
Sample collection: Samples are collected from the uterine cavity using minimally invasive techniques such as transcervical swabs or aspirates. The collection method may vary depending on the specific study or clinical context.
DNA extraction: The collected samples are processed to extract the microbial DNA. This step involves breaking open the microbial cells and isolating the DNA for further analysis.
Library preparation: The extracted DNA is then subjected to library preparation, which involves the addition of specific adapters and barcodes to the DNA fragments. These barcodes allow for multiple samples to be sequenced together in a single sequencing run.
Sequencing: The prepared DNA libraries are loaded onto a next-generation sequencing platform, such as Illumina or Ion Torrent. The sequencing platform generates millions of short DNA sequences, known as reads, from the DNA fragments in the library.
Bioinformatics analysis: The sequenced reads are then processed through bioinformatics pipelines to analyse and interpret the data. This analysis involves mapping the reads to reference microbial genomes or databases to identify the microorganisms present in the samples. It also includes assessing the relative abundances of different microbial species and analysing the diversity and composition of the uterine microbiota.
NGS-based uterine microbiome testing offers several advantages over traditional culture-based methods, as it allows for the identification of a wide range of microorganisms, including bacteria, viruses, fungi, and other microbes. It also provides a more comprehensive view of the microbial community and can detect low-abundance microorganisms that may be missed by culture-based techniques.
3 important ways
microbiota testing
may benefit you
Uterine microbiota testing, exams the microbial composition present in the uterus. Until recently it was considered a sterile environment but research shows that it harbors its own microbial community.
Here are 3 important reasons why microbiota testing is a valuable clinical tool and could be beneficial for you...

Your reproductive health
E.g. endometritis, infertility, preterm birth ...
The uterine microbiome plays a crucial role in maintaining reproductive health. Changes in microbial composition is associated with conditions such as endometritis, infertility, preterm birth, and certain gynecological disorders.
Uterine microbiota testing can help identify imbalances (dysbiosis) and aid in the diagnosis and management of these conditions.

Improved fertility treatment success
optimised uterine environment
The uterine environment plays a vital role in successful implantation of an embryo during pregnancy.
Imbalances in the uterine microbiome have been associated with reduced fertility and lower success rates of ART procedures, including in vitro fertilization (IVF).
Testing of microbiota can aid personalised fertility treatments to optimise the uterine environment for successful implantation and pregnancy.

Individualised fertility treatment
Unique microbial composition
We all have unique microbial composition, and this extends to the uterine microbiome in women.
By characterising an individual's uterine microbiota, doctors can gain specific insights into their reproductive health and make informed decisions regarding treatment strategies.
This approach can improve outcomes by tailoring interventions for specific microbial imbalances.
Reproductive immunology and microbiota testing
THE SPOTLIGHT IS ON
Interplay of the immune system and the uterine microbiome
The spotlight is on the uterine microbiome and unpicking the mechanisms by which it affects fertility and the immune system. Understanding their interactions has solid implications for fertility optimisation and assisted reproductive technologies.
Microbiome-immune interPLAY
The uterine microbiome interacts with the local immune system, influencing immune responses and tolerance during pregnancy. Researchers are currently exploring this relationship to better understand their impact on reproductive processes.
IMMUNE MODULATION
The uterine microbiome plays a pivotal role in modulating the immune system. NGS-based testing can identify specific microbial taxa associated with immune regulation or inflammation and researchers can gain insights into mechanisms of immune modulation in reproduction.
Impact on Fertility
The uterine microbiome has been implicated in infertility, implantation failure, preterm birth, and pregnancy complications. Uterine microbiome testing can help identify microbial biomarkers associated with immune-related reproductive disorders and inform potential diagnostic or therapeutic strategies.
Inflammation and dysbiosis
Imbalance in the uterine microbiome (Dysbiosis) has also been associated with inflammatory conditions such as endometritis. Microbiome testing can help identify specific microbial patterns associated with these conditions which aids our understanding of immunological reactions and allows for targeted treatment.
By identifying microbial factors that influence immune responses, researchers can explore potential interventions aimed at modulating the microbiome-immune axis to improve fertility outcomes and increase the success rates of fertility treatments.

About us
Life Clinic is an infertility, IVF and Gynaecological Clinic in Athens, Greece, with top success rates and specialisation in immunological disorders and unexplained infertility.
We offer IVF, Egg Donation, Embryo Donation, Egg freezing, PGD/PGS and extensive investigation, treatment and monitoring for the immunological disorders that affect fertility.
Life Clinic is lead by Dimitri Papanikolaou, Obstetrician Gynaecologist specialised in Reproductive Medicine and Reproductive Immunology.
